Tissue-resident Memory T cells as Early Responders to Cancer Immunotherapy
See manuscript in Cell (2022)
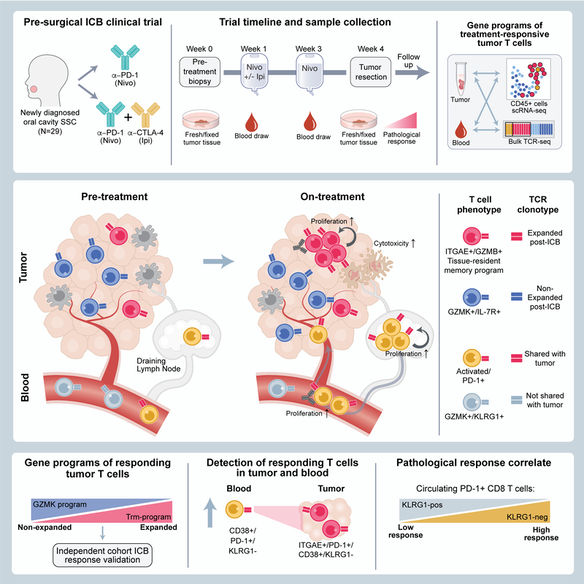
Neoadjuvant immune checkpoint blockade has shown promising clinical activity. Here, we characterized early kinetics in tumor-infiltrating and circulating immune cells in oral cancer patients treated with neoadjuvant anti-PD-1 or anti-PD-1/CTLA-4 in a clinical trial (NCT02919683).Tumor-infiltrating CD8 T cells that clonally expanded during immunotherapy expressed elevated tissue-resident memory and cytotoxicity programs, which were already active prior to therapy, supporting the capacity for rapid response. Systematic target discovery revealed that treatment-expanded tumor T cell clones in responding patients recognized several self-antigens, including the cancer-specific antigen MAGEA1.Treatment also induced a systemic immune response characterized by expansion of activated T cells enriched for tumor-infiltrating T cell clonotypes, including both pre-existing and emergent clonotypes undetectable prior to therapy. The frequency of activated blood CD8 T cells, notably pre-treatment PD-1-positive KLRG1-negative T cells, was strongly associated with intra-tumoral pathological response. These results demonstrate how neoadjuvant checkpoint blockade induces local and systemic tumor immunity.
See manuscript in Cell (2022)
Neoadjuvant immune checkpoint blockade has shown promising clinical activity. Here, we characterized early kinetics in tumor-infiltrating and circulating immune cells in oral cancer patients treated with neoadjuvant anti-PD-1 or anti-PD-1/CTLA-4 in a clinical trial (NCT02919683).Tumor-infiltrating CD8 T cells that clonally expanded during immunotherapy expressed elevated tissue-resident memory and cytotoxicity programs, which were already active prior to therapy, supporting the capacity for rapid response. Systematic target discovery revealed that treatment-expanded tumor T cell clones in responding patients recognized several self-antigens, including the cancer-specific antigen MAGEA1.Treatment also induced a systemic immune response characterized by expansion of activated T cells enriched for tumor-infiltrating T cell clonotypes, including both pre-existing and emergent clonotypes undetectable prior to therapy. The frequency of activated blood CD8 T cells, notably pre-treatment PD-1-positive KLRG1-negative T cells, was strongly associated with intra-tumoral pathological response. These results demonstrate how neoadjuvant checkpoint blockade induces local and systemic tumor immunity.
Analysis of immune responses to pre-surgical immunotherapy
Inflammatory Adverse Events of Cancer Immunotherapy
See manuscript in Cell (2020) and review in Cell (2021)
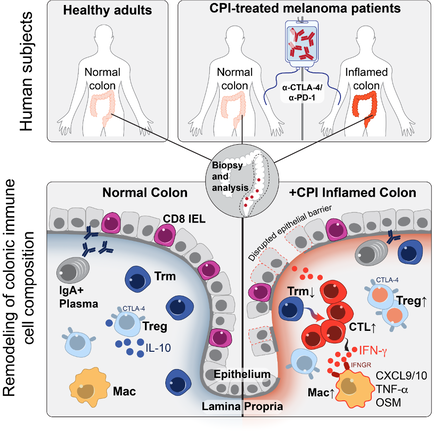
Checkpoint blockade with antibodies specific for the PD-1 and CTLA-4 inhibitory receptors can induce durable responses in a wide range of human cancers, but immune-related adverse events (irAEs) represent a major challenge for the field. The immunological mechanisms of irAEs have been difficult to study because colitis and other inflammatory side effects are not observed in murine tumor models treated with checkpoint inhibitors. We are investigating the cellular and molecular pathways using colitis as an important example because it is a common and frequently severe adverse event. We are studying recently diagnosed colitis patients who have not yet received drug treatment for this irAE and use diagnostic tissue biopsies for a comprehensive analysis of immune cell populations by single-cell RNA-sequencing (scRNA-seq) and multi-spectral flow cytometry. This approach had led to the discovery of cellular and molecular pathways in colitis as well as the identification of potential therapeutic targets for this important side effect of cancer immunotherapy.
See manuscript in Cell (2020) and review in Cell (2021)
Checkpoint blockade with antibodies specific for the PD-1 and CTLA-4 inhibitory receptors can induce durable responses in a wide range of human cancers, but immune-related adverse events (irAEs) represent a major challenge for the field. The immunological mechanisms of irAEs have been difficult to study because colitis and other inflammatory side effects are not observed in murine tumor models treated with checkpoint inhibitors. We are investigating the cellular and molecular pathways using colitis as an important example because it is a common and frequently severe adverse event. We are studying recently diagnosed colitis patients who have not yet received drug treatment for this irAE and use diagnostic tissue biopsies for a comprehensive analysis of immune cell populations by single-cell RNA-sequencing (scRNA-seq) and multi-spectral flow cytometry. This approach had led to the discovery of cellular and molecular pathways in colitis as well as the identification of potential therapeutic targets for this important side effect of cancer immunotherapy.
Major molecular and cellular changes in the colon mucosa in patient with checkpoint blockade induced colitis
We observed a striking accumulation of CD8 T cells with highly cytotoxic and proliferative states and no evidence of regulatory T cell depletion. T cell receptor (TCR) sequence analysis demonstrated that a substantial fraction of colitis-associated CD8 T cells originated from tissue-resident memory T cell populations, explaining the frequently early onset of colitis symptoms following treatment initiation. Our analysis also identified cytokines, chemokines and surface receptors that could serve as therapeutic targets for colitis and potentially other inflammatory side effects of checkpoint blockade.
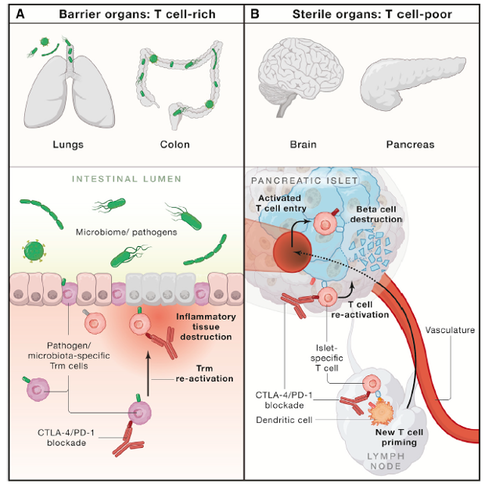
Proposed functional subgrouping of inflammatory toxicities caused by checkpoint blockade
We are now performing mechanistic analyses in a clinical trial that evaluates a major inflammatory pathway we identified in the single cell studies. We will use pre- and on-treatment biopsies to define pathways of drug responsiveness and resistance. This effort provides an opportunity to study dynamic changes in immune microenvironments in human tissues in response to therapy.
Luoma, A. M., Suo, S., Wang, Y., Gunasti, L., Porter, C. B. M., Nabilsi, N., Tadros, J., Ferretti, A. P., Liao, S.,Gurer, C., Chen, Y.-H., Criscitiello, S., Ricker, C. A., Dionne, D., Rozenblatt-Rosen, O., Uppaluri, R.,Haddad, R. I., Ashenberg, O., Regev, A., Van Allen, E. M., MacBeath, G., Schoenfeld, J. D., and Wucherpfennig, K. W. (2022) Tissue-resident memory and circulating T cells are early responders to pre-surgical cancer immunotherapy. Cell advance online publication, doi: 10.1016/j.cell.2022.06.018
Luoma, A. M., Suo, S., Williams, H. L., Sharova, T., Sullivan, K., Manos, M., Bowling, P., Hodi, F. S., Rahma, O., Sullivan, R. J., Boland, G. M., Nowak, J. A., Dougan, S. K., Dougan, M., Yuan, G. C., and Wucherpfennig, K. W. (2020) Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 182, 655-671 e622
Dougan, M., Luoma, A. M., Dougan, S. K., and Wucherpfennig, K. W. (2021) Understanding and treating the inflammatory adverse events of cancer immunotherapy, Cell 2021, 184: 1575-1588.
Luoma, A. M., Suo, S., Williams, H. L., Sharova, T., Sullivan, K., Manos, M., Bowling, P., Hodi, F. S., Rahma, O., Sullivan, R. J., Boland, G. M., Nowak, J. A., Dougan, S. K., Dougan, M., Yuan, G. C., and Wucherpfennig, K. W. (2020) Molecular Pathways of Colon Inflammation Induced by Cancer Immunotherapy. Cell 182, 655-671 e622
Dougan, M., Luoma, A. M., Dougan, S. K., and Wucherpfennig, K. W. (2021) Understanding and treating the inflammatory adverse events of cancer immunotherapy, Cell 2021, 184: 1575-1588.